- Follow on Twitter
- Follow our RSS Feed
- Share this page


The main purpose of Phase III trials (often called confirmatory trials) is to demonstrate efficacy of the treatment under study in a specific population that has a given disease [1]. The drug tested is compared to a control treatment (could be either placebo or standard-of-care) and patients are in most cases randomised. Such a trial should provide the definite proof of treatment superiority (or equivalence or non-inferiority, depending on the purpose of the trial). The sample sizes are often large, varying from a couple of hundred to even tens of thousands of patients. They also aim to identify long-term or rare side effects that have not been discovered in the previous phases. The aim of a Phase III trial is to provide sufficient evidence for the drug to be licensed by a regulatory agency for use in patients. However, nowadays only 25-30% of treatments gets approved and make it to patients [1].
A general introduction of how the followings questions are answered can be found here: General Introduction on Drug Development
1. What are the responsibilities of a statistician in this phase?
The statistician is responsible for designing and analysing the trial. The design stage involves making decisions on questions like the sample size (the number of patients involved), the study design, the randomisation strategy (how the patients should be allocated to the different treatment options), the choice of endpoints (how the effect of the drug can be measured) and the statistical analysis methods. These questions might be discussed with methodological experts and health authorities. Then, the statistician writes the details into the statistical part of the study protocol and writes a separate document known as the Statistical Analysis Plan (SAP). During the study, the statistician has to monitor the trial (e.g., check if the randomization is being adhered to). After the trial is finished, the analysis of the trial data according to the SAP is performed. This involves not only the calculations that are necessary to decide if the new drug is better than, or as efficient, as the already approved drug or placebo treatment (primary endpoint), but also, for example, the validation of the data. Finally, all the statistical results are described in the clinical study report.
2. With whom do statisticians collaborate in this phase?
The most important collaboration partners in this phase are data managers and clinicians. Other important partners are statistical functions such as programmers, other trial statisticians, pharmacokinetic modelers and methodologists. Additional non-statistical collaborators are regulatory experts, funders, trial managers, ethicists, commercial teams, clinical submission teams and drug supply specialists.
3. What are the major challenges emerging from these collaborations?

It is most difficult to communicate with non-statistical collaborators because the collaborators might be speaking a “different language”. Clinicians might have other priorities, approaches and concerns and it is important that the statistician makes him or herself understood and ensures that medical staff are approaching the research questions from the same perspective as the statisticians. An important role of the statistician is to translate the medical questions into statistically testable hypotheses. As an example, the pre-specification of the primary analysis may be difficult to communicate because the uncertainty at the design stage of the trial might be high and it is not easy to agree on an assumed treatment effect beforehand.
4. What are major ethical challenges in this phase?

The main ethical issue is whether it is ethically reasonable to use placebo for the control group. In case of comparing the test treatment with placebo, a design that keeps the rate of the patients receiving placebo lower than the rate of patients in the group with the test treatment is often required. Consequently, one mandatory challenge is the sample size calculation so that the ethics committees know the number of patients that are planned to be treated in the trial. Further critical issues are related to randomisation (e.g., the choice of the control group, the degree of blinding). The above challenges can be summarised in finding the situation under which randomisation can be ethically used. In addition, one ethical challenge around statistical analysis might be to interpret the outcome adequately in order to avoid wrong decisions leading to any risks for patients.
5. What are the major statistical challenges in this phase?

The statistician is faced with challenging statistical aspects when designing and analysing the trial. At the design stage, difficulties might include the calculation of sample size, choosing sensible endpoints and finding an appropriate study design. During the analysis procedure, challenges can arise in dealing with missing data, using covariate information adequately, controlling the rate of type I error (wrong rejection of the null hypothesis, i.e., the consumer’s risk) and issues around interim analyses (e.g., deciding in which case the trial can be stopped based on the analysis of interim data). Further statistical problems might occur in complex trials with multiple testing.
6. Which innovative study designs are particularly important in this phase?
The adaptive design is considered as a highly innovative study design due to its flexibility and feasibility. In general, an adaptive (in design) clinical trial is a trial with a prescribed opportunity for modifications as the study progresses (e.g., in sample size, in dosage, in the hypothesis testing) based on analysis of interim data. Other innovations to the standard RCT (randomised controlled trial in which the subjects or groups of the subjects are randomly allocated) are the biomarker-marked designs (e.g., enrichment design) and multi-arm designs. The group sequential design also represents a ground-breaking design, as its main characteristic is the opportunity to stop the trial for futility or efficacy. However, the major scope for design innovations seems to be in Phase II.
7. Which statistical analyses are most important in this phase?
The most important statistical methods seem to be the implementation of multiple testing (where a set of hypotheses are to be tested at the same time) and interim analyses (e.g., interpretation of early-stage results in the trial). In addition, survival analysis (e.g., analysing data where the time until the occurrence of an event such as death is observed) is considered as relevant, in particular related to the Kaplan-Meier method for estimating survival functions. Another relevant method is the regression analysis such as logistic regression (where the dependent variable representing the outcome of interest is categorical) and mixed model (where both random effects and fixed effects are included). Furthermore, methods for missing data as well as methods based on Bayesian approaches are also important for Phase III.
8. How often are Bayesian methods used?
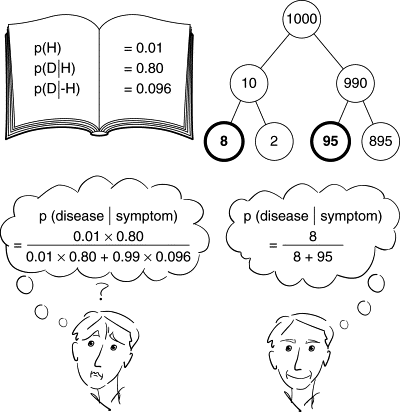
Bayesian methods are sometimes (but rarely) used in Phase III trials. They are more applicable in earlier phase trials as Phase III trials are centered on an independent confirmation of treatment benefit. Interim decision making in Phase III can include Bayesian elements, however, the health authorities and other stakeholders insist that the ultimate test of treatment benefit has to be an isolated one from this trial. In addition, if Bayesian methodology is used, the frequentist operating characteristics of such designs should be thoroughly examined. The methods are used as sensitivity analysis, but rather not as the primary efficacy analysis.
On the other hand, Bayesian methodology could have potential in trials in rare diseases, where frequentist sample size requirements might be unrealistically high. Bayesian methods could also be used to complement frequentist analyses, to support decision making, to address a missing data issue or for computing predictive power to compare design options. It is argued that the potential for using Bayesian methodology is great but the reality is often disappointing [2-3].
9. What are the statistical topics that are particularly “hot” in this phase?

Particularly ‘hot’ topics in Phase III include missing data and estimands that are connected with this issue. Estimands help to address the problem by posing the correct research question [4]. Another area of research that has attracted a lot of attention is targeted therapy and evaluation of treatment effect based on biomarkers and the topic of subgroup analysis. Furthermore, Bayesian methods or novel designs such as adaptive, flexible or seamless Phase II/III trials are important. Non-proportional hazards, reproducibility, recurrent events, small populations and meeting primary endpoint are also areas of research.
10. Are newly published methods used regularly in practice?
The opinions about newly published methods being regularly used in practice are divided. The reasons for lack of use of them could include complexity of such methodology, skepticism and a reluctance of the health authorities to accept it. Furthermore, understanding implications of newly published methods is a long process, and usually there is lack of training on such methods as well as lack of validation.
11. What is the connection between Phase II and Phase III studies?
 In general, Phase III and IV studies are not as strongly connected as the previous phases of drug development. However, there exist some examples of connections. In Phase III trials information on serious adverse events is collected, which may be useful in planning pharmacovigilance studies in Phase IV which are conducted after the drug is approved. Furthermore, Phase IV studies may be useful for collecting additional data that might have been requested by the authorities after conducting a Phase III study. Phase III studies can also be vital for the progression of the compound in post marketing or new indications, or in the creation of new hypotheses, potentially on secondary endpoints that help to better characterise the properties of the treatment and patient population that benefits most from it.
In general, Phase III and IV studies are not as strongly connected as the previous phases of drug development. However, there exist some examples of connections. In Phase III trials information on serious adverse events is collected, which may be useful in planning pharmacovigilance studies in Phase IV which are conducted after the drug is approved. Furthermore, Phase IV studies may be useful for collecting additional data that might have been requested by the authorities after conducting a Phase III study. Phase III studies can also be vital for the progression of the compound in post marketing or new indications, or in the creation of new hypotheses, potentially on secondary endpoints that help to better characterise the properties of the treatment and patient population that benefits most from it.
“This blog was written by Enya, Johanna and Julia”

References:
Leave a Reply
You must be logged in to post a comment.